Chapter 1 of Class 11 Physics – 'Units and Measurements.' This foundational chapter delves into the essence of precise scientific understanding, introducing the International System of Units and essential measurement tools. Elevate your learning experience with the comprehensive resources available through CBSE NCERT Download, ensuring a holistic grasp of fundamental concepts for academic excellence.
Chapter 1 – Units and Measurement in Physics CBSE OF Class 11 NCERT DOWNLOAD
UNITS IN PHYSICS
In physics, units are standardised measures used to express and quantify physical quantities. The choice of units is essential for clear communication and accurate representation of measurements. There are two types of units:
-
Base Units

-
-
These are fundamental units representing basic physical quantities. The International System of Units (SI) defines seven base units:
-
Metre (m): Unit of length
-
Kilogram (kg): Unit of mass
-
Second (s): Unit of time
-
Ampere (A): Unit of electric current
-
Kelvin (K): Unit of temperature
-
Mole (mol): Unit of amount of substance
-
Candela (cd): Unit of luminous
-
-
-
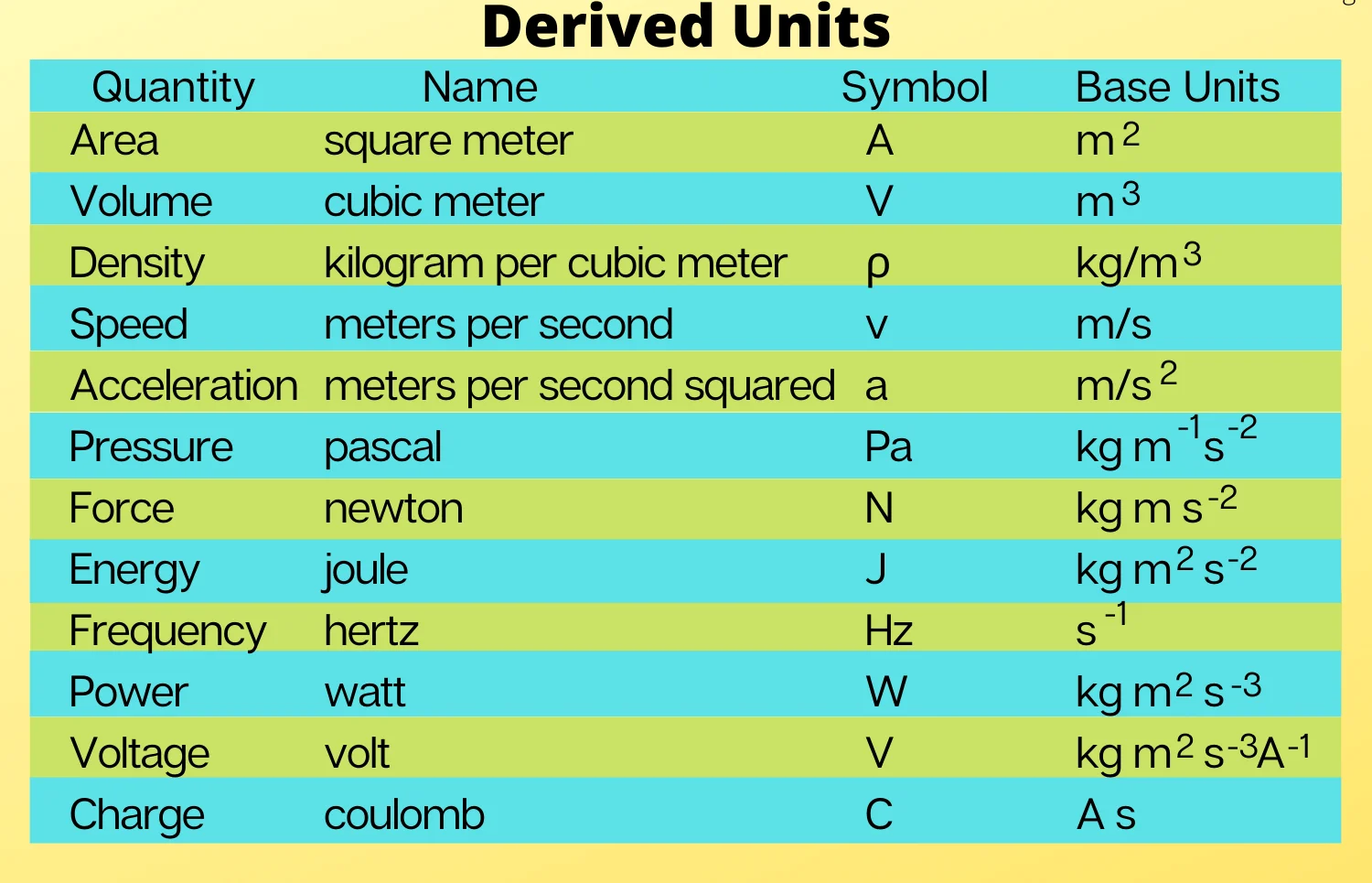
Derived Units:
-
These are units derived from combinations of base units. Examples include
-
Newton (N): Unit of force (kg·m/s²)
-
Joule (J): Unit of energy (kg·m²/s²)
-
Watt (W): Unit of power (J/s)
-
Coulomb (C): Unit of electric charge
-
-
What is the INTERNATIONAL SYSTEM OF UNITS?


The International System of Units (SI) is the modern form of the metric system and is the world's most widely used system of measurement. It provides a standardised and coherent framework for expressing physical quantities. The SI was established to overcome the inconsistencies and variety of measurement systems used across different countries and scientific disciplines.
Key features of the SI include
Base Units: The SI defines seven base units, from which all other units are derived. These are the metre (m), kilogram (kg), second (s), ampere (A), kelvin (K), mole (mol), and candela (cd). Each base unit represents a fundamental physical quantity.
Derived Units: These are units derived from combinations of the base units. For example, the unit of speed is metres per second (m/s), which combines the base units of length and time.
Prefixes: The SI system uses prefixes to denote multiples or fractions of the base units. For instance, kilo- (k) represents a factor of 1000, so a kilogram is 1000 grams.
Consistency: The SI system is designed to be consistent and coherent, meaning that the relationships between different units are logical and mathematically straightforward.
International Acceptance: The SI system is widely adopted and recognized globally, providing a common language for scientists, engineers, and people in various fields.
Benefits of INTERNATIONAL SYSTEM OF UNITS
Global Standardisation: The SI provides a globally recognized and consistent system of measurement. This standardisation facilitates communication and collaboration among scientists, engineers, and researchers worldwide.
Ease of Learning: The SI is designed with simplicity in mind, making it easy to learn and use. The system's straightforward structure and logical relationships between units contribute to its user-friendly nature.
Coherence: The SI is a coherent system, meaning that the relationships between different units are logical and mathematically consistent. This coherence simplifies mathematical calculations and conversions between different units.
Flexibility: The SI is adaptable and flexible, accommodating a wide range of scientific disciplines and applications. It provides a framework for measuring diverse quantities, from the microscopic scale in physics to the macroscopic scale in engineering.
Interdisciplinary Application: The SI is applicable across various scientific and engineering disciplines, fostering interdisciplinary collaboration. This versatility allows for a seamless exchange of information and ideas.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. What is the International System of Units (SI)?
-
Answer: The International System of Units, abbreviated as SI from the French "Système International d'Unités," is the globally accepted system of measurement used in science, industry, and everyday life. It establishes a standardized set of units for various physical quantities.
Q2. Why was the SI System Introduced?
-
Answer: The SI system was introduced to provide a uniform and consistent way of measuring physical quantities, fostering international collaboration in science and commerce. It aims to eliminate confusion caused by different measurement systems.
Q3. What are the Seven Base SI Units?
-
Answer: The seven base SI units are:
-
Meter (m) – for length
-
Kilogram (kg) – for mass
-
Second (s) – for time
-
Ampere (A) – for electric current
-
Kelvin (K) – for temperature
-
Mole (mol) – for amount of substance
-
Candela (cd) – for luminous intensity
-
Q4. How are SI Units Derived?
-
Answer: SI units are derived from combinations of the base units. For example, the unit for speed is meters per second (m/s), which combines the base units for length and time.
Q5. What is the Importance of Standardization in Measurements?
-
Answer: Standardization ensures consistency and accuracy in measurements. It allows scientists, engineers, and individuals globally to communicate and collaborate effectively, as everyone uses the same units and standards.

| CBSE CLASS 11th |
| Physics Chapters |
| Chapter 1 : UNITS AND MEASUREMENTS |
| 1.1 Introduction |
| 1.3 Significant figures |
| 1.4 Dimensions of physical quantities |
| 1.5 Dimensional formulae and dimensional equations |
| 1.6 Dimensional analysis and its applications |
| Chapter 3 : MOTION IN A PLANE |
| Chapter 4 : LAWS OF MOTION |
| Chapter 5 : WORK, ENERGY AND POWER |
| Chapter 6 : SYSTEM OF PARTICLES AND ROTATIONAL MOTION |
| Chapter 7 : GRAVITATION |
| Chapter 8: MECHANICAL PROPERTIES OF SOLIDS |
| Chapter 9: MECHANICAL PROPERTIES OF FLUIDS |
| Chapter 10: THERMAL PROPERTIES OF MATTER |
| Chapter 12: KINETIC THEORY |
| Chapter 13: OSCILLATIONS |
| Chapter 14: WAVES |
| Chemistry Chapters |
| Chapter 1: SOME BASIC CONCEPTS OF CHEMISTRY |
| Chapter 2 : STRUCTURE OF ATOMS |
| Chapter 3: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES |
| Chapter 4 : CHEMICAL BONDING AND MOLECULAR STRUCTURE |
| Chapter 5 : THERMODYNAMICS |
| Chapter 6 : EQUILIBRIUM |
| Chapter 7: REDOX REACTIONS |
| Chapter 8 : ORGANIC CHEMISTRY – SOME BASIC PRINCIPLE AND TECHNIQUES |
| Chapter 9: Hydrocarbons HYDROCARBONS |
| Mathematics chapter |
| Chapter 1. SETS |
| Chapter 2. RELATIONS AND FUNCTIONS |
| Chapter 3. TRIGONOMETRIC FUNCTIONS |
| Chapter 4. COMPLEX NUMBER AND QUADRATIC EQUATIONS |
| Chapter 5. LINEAR INEQUALITIES |
| Chapter 6. PERMUTATIONS AND COMBINATIONS |
| Chapter 7. BINOMIAL THEOREM |
| Chapter 8. SEQUENCES AND SERIES |
| Chapter 9. STRAIGHT LINES |
| Chapter 10. CONIC SECTIONS |
| Chapter 11. INTRODUCTION TO THREE-DIMENSIONAL GEOMETRY |
| Chapter 12. LIMITS AND DERIVATIVES |
| Chapter 13. STATISTICS |
| Chapter 14. PROBABILITY |
| Class 8 Link soon |
| Class 9 Link soon |
| Class 10 Link soon |
| Class 12 Link soon |
Leave a Reply