The study of atomic models stands as a testament to human curiosity and the relentless pursuit of knowledge. From ancient philosophical ponderings to the cutting-edge theories of today, the concept of the atom has evolved, revealing a fascinating tapestry of understanding. As we embark on this intellectual voyage, we will traverse through the diverse models that have shaped our perception of the smallest units of matter. The journey begins with Dalton's simple yet revolutionary idea of indivisible atoms, paving the way for a new era in chemistry. We'll witness the visionary leaps of Thomson, Rutherford, and Bohr, each introducing novel concepts that refine our understanding of atomic structure. The narrative unfolds further as quantum mechanics takes the stage, ushering in a probabilistic realm where electrons exist as waves within defined orbitals.
A Comprehensive Guide to Atomic Models in CBSE NCERT Download
What are atomic models?
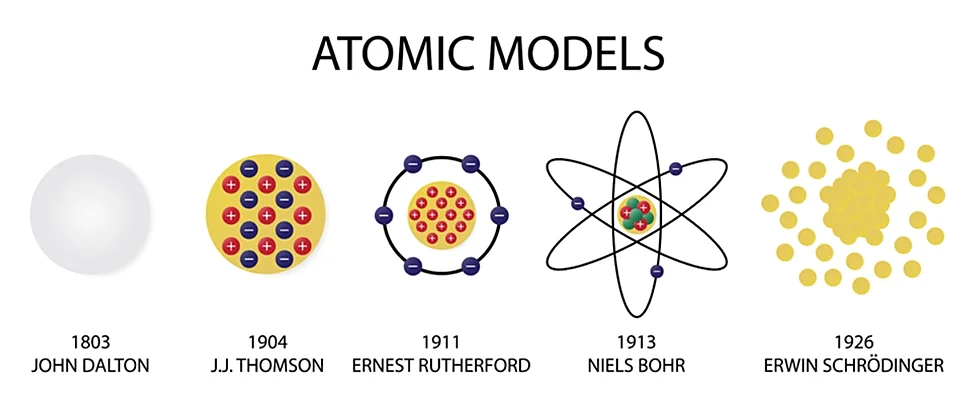
Atomic models are conceptual representations that describe the structure and behavior of atoms—the basic building blocks of matter. These models have evolved over time as our understanding of atomic structure has advanced. Each atomic model reflects the prevailing scientific knowledge and theories of its era. Here is an overview of some key atomic models:
-
Dalton's Model (1803): John Dalton proposed the idea that atoms are indivisible and indestructible spheres. This model laid the groundwork for the concept of the atom as a fundamental unit of matter.
-
Thomson's Model (1897): J.J. Thomson's discovery of the electron led to the "plum pudding" model, where electrons were thought to be embedded in a positively charged "pudding" or matrix. This model depicted atoms as having a positive charge overall.
-
Rutherford's Model (1911): Ernest Rutherford's gold foil experiment revealed that atoms have a small, dense nucleus containing positively charged protons. Electrons orbit the nucleus at a distance. This model is often referred to as the nuclear model.
-
Bohr's Model (1913): Niels Bohr proposed a model where electrons move in fixed orbits around the nucleus. Electrons can only exist in specific energy levels, and transitions between these levels give rise to spectral lines.
-
Quantum Mechanical Model (1920s onward): Developed from the mid-1920s onward, this model, based on quantum mechanics, describes electrons as existing within probability distributions called orbitals. It incorporates wave-particle duality and the uncertainty principle.
These models are not mutually exclusive; rather, they represent stages in the development of atomic theory. The Quantum Mechanical Model, in particular, is the current paradigm, providing a sophisticated understanding of atomic behavior. It describes electrons not as discrete particles with fixed paths but as probability distributions in regions around the nucleus known as orbitals. This model has been successful in explaining a wide range of atomic phenomena and is fundamental to modern chemistry and physics.
What is Atomic Theory?
Atomic theory is a scientific model that explains the nature and behavior of matter at the atomic level. It represents a fundamental concept in chemistry and physics and has evolved over centuries as our understanding of the structure of atoms has deepened. The key components of atomic theory include:
-
Indivisibility of Atoms (Dalton): John Dalton's atomic theory, proposed in the early 19th century, suggested that atoms are indivisible and indestructible particles, representing the basic building blocks of matter.
-
Discovery of Subatomic Particles: Subsequent advancements, such as the discovery of electrons by J.J. Thomson and the identification of the nucleus by Ernest Rutherford, revealed that atoms are composed of smaller subatomic particles, including protons, neutrons, and electrons.
-
Quantum Mechanics: The development of quantum mechanics in the early 20th century, including the work of Schrödinger and Heisenberg, brought about a more sophisticated understanding of the behavior of electrons within atoms. It introduced the concept of electron probability clouds and the idea of quantized energy levels.
-
Modern Atomic Theory: The modern atomic theory incorporates principles of quantum mechanics and describes atoms as consisting of a nucleus containing protons and neutrons, surrounded by electrons in specific energy levels or orbitals. This theory accounts for the probabilistic nature of electron behavior and forms the basis of contemporary atomic understanding.
The atomic theory serves as a foundation for explaining chemical reactions.
What is Atomic Structure?
Atomic structure refers to the organization and arrangement of subatomic particles within an atom. At the nucleus's core reside positively charged protons and neutrally charged neutrons, while negatively charged electrons orbit in specific energy levels or orbitals. The number of protons defines an element's identity, determining its place on the periodic table. This intricate framework, governed by quantum mechanics, reveals the probabilistic nature of electron positions. Understanding atomic structure is fundamental to comprehending chemical bonding, the periodicity of elements, and the diverse properties of matter, contributing to advancements in chemistry, physics, and various scientific disciplines.
What is Atomic Mass?
Atomic mass is the mass of an atom, expressed in atomic mass units (amu). It represents the sum of the masses of protons, neutrons, and electrons in an atom. Protons and neutrons contribute significantly to the atomic mass, while electrons, due to their much smaller mass, have a negligible impact. The atomic mass is crucial in chemistry, as it serves to calculate the molar mass of elements, facilitating stoichiometric calculations in reactions. It is usually found on the periodic table beneath the symbol of each element and helps determine the average mass of isotopes considering their abundance in nature.
What is an Atomic Number?
The atomic number is a fundamental property of an element, denoted by the number of protons in the nucleus of an atom. It uniquely defines the element, determining its chemical properties and position on the periodic table. The atomic number is crucial for identifying elements, as elements with different atomic numbers have distinct characteristics. It plays a pivotal role in establishing electron configurations and guiding the arrangement of electrons in energy levels. The periodic table is organized based on increasing atomic number, showcasing the elements' natural order and facilitating our understanding of the relationships between them in terms of reactivity and properties.
CBSE Class 11th Downloadable Resources:
|
1. CBSE Class 11th Topic Wise Summary |
View Page / Download |
|
2. CBSE Class 11th NCERT Books |
View Page / Download |
|
3. CBSE Class 11th NCERT Solutions |
View Page / Download |
|
4. CBSE Class 11th Exemplar |
View Page / Download |
|
5. CBSE Class 11th Sample Papers |
View Page / Download |
|
6. CBSE Class 11th Question Bank |
View Page / Download |
|
7. CBSE Class 11th Topic Wise Revision Notes |
View Page / Download |
|
8. CBSE Class 11th Last Minutes Preparation Resources |
View Page / Download |
|
9. CBSE Class 11th Best Reference Books |
View Page / Download |
|
10. CBSE Class 11th Formula Booklet |
View Page / Download |
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. What does the atomic number determine?
Answer. The atomic number determines an element's position on the periodic table and its chemical properties.
Q2. How is the periodic table organized based on atomic number?
Answer. The elements are arranged in ascending order of atomic number in the periodic table.
Q3.Is the atomic number the same for all isotopes of an element?
Answer. Yes, the atomic number remains constant for all isotopes of an element, as it is defined by the number of protons.
Q4.How can I find the number of neutrons using the atomic number?
Answer. Subtract the atomic number from the atomic mass (rounded to the nearest whole number) to find the number of neutrons.
Q5.How does the atomic number relate to electron configuration?
Answer. The atomic number determines the number of electrons in an atom, influencing its electron configuration and energy levels.

| CBSE CLASS 11th Chemistry Chapters |
| Chapter1: SOME BASIC CONCEPTS OF CHEMISTRY |
| Chapter2: STRUCTURE OF ATOMS |
| > Discovery of Sub-Atomic Particle |
| > Atomic Models |
| > Developments Leading to the Bohr’s Model of Atom |
| > Bohr’s Model for Hydrogen Atom |
| > Quantum Mechanical Model of Atom |
| Chapter3: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES |
| Chapter4: CHEMICAL BONDING AND MOLECULAR STRUCTURE |
| Chapter5: THERMODYNAMICS |
| Chapter6: EQUILIBRIUM |
| Chapter7: REDOX REACTIONS |
| Chapter8: ORGANIC CHEMISTRY – SOME BASIC PRINCIPLE AND TECHNIQUES |
| Chapter9: Hydrocarbons HYDROCARBONS |
| CBSE Class 11 Physics Chapters |
| Chapter1: UNITS AND MEASUREMENTS |
| Chapter2: MOTION IN A STRAIGHT LINE |
| Chapter3: MOTION IN A PLANE |
| Chapter4: LAWS OF MOTION |
| Chapter5: WORK, ENERGY AND POWER |
| Chapter6: SYSTEM OF PARTICLES AND ROTATIONAL MOTION |
| Chapter7: GRAVITATION |
| Chapter8: MECHANICAL PROPERTIES OF SOLIDS |
| Chapter9: MECHANICAL PROPERTIES OF FLUIDS |
| Chapter10: THERMAL PROPERTIES OF MATTER |
| Chapter12: KINETIC THEORY |
| Chapter13: OSCILLATIONS |
| Chapter14: WAVES |
| CBSE Class 11 Mathematics chapter |
| Chapter1: SETS |
| Chapter2: RELATIONS AND FUNCTIONS |
| Chapter3: TRIGONOMETRIC FUNCTIONS |
| Chapter4: COMPLEX NUMBER AND QUADRATIC EQUATIONS |
| Chapter5: LINEAR INEQUALITIES |
| Chapter6: PERMUTATIONS AND COMBINATIONS |
| Chapter7: BINOMIAL THEOREM |
| Chapter8: SEQUENCES AND SERIES |
| Chapter9: STRAIGHT LINES |
| Chapter10: CONIC SECTIONS |
| Chapter11: INTRODUCTION TO THREE-DIMENSIONAL GEOMETRY |
| Chapter12: LIMITS AND DERIVATIVES |
| Chapter13: STATISTICS |
| Chapter14: PROBABILITY |
| Class 8 Link soon |
| Class 9 Link soon |
| Class 10 Link soon |
| Class 12 Link soon |
Leave a Reply