The discovery of subatomic particles marked a transformative epoch in our understanding of the fundamental building blocks of matter. From the enigmatic electron to the resilient quark, this journey through the subatomic landscape unravels the mysteries that connect the microcosm to the macrocosm. Join us as we embark on a historical odyssey, tracing the footsteps of visionary scientists who, armed with curiosity and ingenious experiments, uncovered a realm where particles dance in ways that defy the limits of classical intuition. This exploration transcends the visible and propels us into a universe where particles reveal their secrets, shaping the very fabric of our understanding of the physical world. Welcome to the captivating saga of the discovery of subatomic particles—a narrative that continues to redefine the boundaries of our cosmic comprehension.
The Fascinating Journey of Subatomic Particle Discovery in CBSE NCERT Download
Discovery of sub-atomic Particle
The discovery of subatomic particles is a fascinating journey that unfolded over the 19th and 20th centuries. Here's a brief overview of key milestones in this discovery:

Discovery of Electrons:
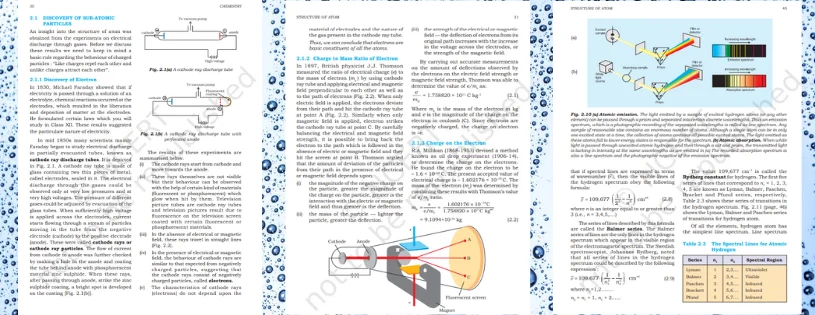
- Cathode Ray Tube Experiments (Late 19th Century):
J.J. Thomson conducted experiments with cathode ray tubes and discovered electrons in 1897. He observed that cathode rays (streams of electrons) were attracted to a positive plate, indicating the presence of negatively charged particles.
Discovery of Protons:
- Gold Foil Experiment (1909):
Ernest Rutherford, along with Hans Geiger and Ernest Marsden, conducted the famous gold foil experiment. They discovered that most of the alpha particles passed through the gold foil, suggesting that atoms have a small, dense nucleus containing positively charged particles (later identified as protons).
Discovery of Neutrons:
- Neutron Hypothesis (1932):
James Chadwick proposed the existence of neutrons to explain the discrepancy in atomic mass. In 1932, he experimentally confirmed the existence of neutrons, neutral particles located in the nucleus, through a series of experiments involving beryllium and alpha particles.
Discovery of Quarks:
- Quark Model (1960s):
Murray Gell-Mann and George Zweig independently proposed the quark model in the 1960s, suggesting that protons and neutrons are composed of smaller particles called quarks. Quarks are elementary particles that come in six types or "flavors."
Discovery of W and Z Bosons:
- Gargamelle Collaboration (1973):
The Gargamelle collaboration at CERN discovered the W and Z bosons, particles responsible for the weak force in particle physics. This discovery provided experimental evidence for the electroweak unification theory.
Discovery of the Top Quark:
- CDF and DZero Experiments (1995):
The top quark, the heaviest known elementary particle, was discovered simultaneously by the CDF and DZero collaborations at Fermilab in 1995.
Discovery of the Higgs Boson:
Large Hadron Collider (2012):
The discovery of the Higgs boson, the last missing particle predicted by the Standard Model, was announced at CERN's Large Hadron Collider in 2012. François Englert and Peter Higgs, who theorized its existence in the 1960s, were awarded the Nobel Prize in Physics in 2013.
These discoveries collectively form the foundation of our understanding of subatomic particles and their role in the intricate fabric of the universe.
What is a sub-atomic particle?
Subatomic particles are particles that are smaller than an atom. At the heart of the atom, there are three primary subatomic particles: protons, neutrons, and electrons.
-
Protons: Positively charged particles found in the nucleus of an atom. The number of protons determines the element of an atom.
-
Neutrons: Neutrally charged particles are also located in the nucleus. Neutrons, along with protons, contribute to the atomic mass.
-
Electrons: Negatively charged particles that orbit the nucleus in electron shells. Electrons are much smaller and lighter than protons and neutrons.
These particles were initially considered elementary, meaning they were thought to be indivisible.
CBSE Class 11th Downloadable Resources:
|
1. CBSE Class 11th Topic Wise Summary |
View Page / Download |
|
2. CBSE Class 11th NCERT Books |
View Page / Download |
|
3. CBSE Class 11th NCERT Solutions |
View Page / Download |
|
4. CBSE Class 11th Exemplar |
View Page / Download |
|
5. CBSE Class 11th Sample Papers |
View Page / Download |
|
6. CBSE Class 11th Question Bank |
View Page / Download |
|
7. CBSE Class 11th Topic Wise Revision Notes |
View Page / Download |
|
8. CBSE Class 11th Last Minutes Preparation Resources |
View Page / Download |
|
9. CBSE Class 11th Best Reference Books |
View Page / Download |
|
10. CBSE Class 11th Formula Booklet |
View Page / Download |
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. How were protons discovered?
Answer. Protons were discovered through the gold foil experiment conducted by Ernest Rutherford in 1909. This experiment revealed the existence of a small, dense nucleus within atoms.
Q2. When and how were neutrons discovered?
Answer. Neutrons were proposed by James Chadwick in 1932 to account for the missing mass in atomic nuclei. He experimentally confirmed their existence through experiments involving beryllium and alpha particles.
Q3. What is the quark model, and who proposed it?
Answer. The quark model, proposed independently by Murray Gell-Mann and George Zweig in the 1960s, suggests that protons and neutrons are composed of smaller particles called quarks.
Q4. How were W and Z bosons discovered, and what do they do?
Answer. The Gargamelle collaboration at CERN discovered W and Z bosons in 1973. These particles mediate the weak force responsible for processes like beta decay.
Q5. What is the top quark, and when was it discovered?
Answer. The top quark is the heaviest known elementary particle. It was discovered simultaneously by the CDF and DZero collaborations at Fermilab in 1995.

| CBSE CLASS 11th Chemistry Chapters |
| Chapter1: SOME BASIC CONCEPTS OF CHEMISTRY |
| Chapter2: STRUCTURE OF ATOMS |
| > Atomic Models |
| > Developments Leading to the Bohr’s Model of Atom |
| > Bohr’s Model for Hydrogen Atom |
| > Towards Quantum Mechanical Model of the Atom |
| > Quantum Mechanical Model of Atom |
| Chapter3: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES |
| Chapter4: CHEMICAL BONDING AND MOLECULAR STRUCTURE |
| Chapter5: THERMODYNAMICS |
| Chapter6: EQUILIBRIUM |
| Chapter7: REDOX REACTIONS |
| Chapter8: ORGANIC CHEMISTRY – SOME BASIC PRINCIPLE AND TECHNIQUES |
| Chapter9: Hydrocarbons HYDROCARBONS |
| CBSE Class 11 Physics Chapters |
| Chapter1: UNITS AND MEASUREMENTS |
| Chapter2: MOTION IN A STRAIGHT LINE |
| Chapter3: MOTION IN A PLANE |
| Chapter4: LAWS OF MOTION |
| Chapter5: WORK, ENERGY AND POWER |
| Chapter6: SYSTEM OF PARTICLES AND ROTATIONAL MOTION |
| Chapter7: GRAVITATION |
| Chapter8: MECHANICAL PROPERTIES OF SOLIDS |
| Chapter9: MECHANICAL PROPERTIES OF FLUIDS |
| Chapter10: THERMAL PROPERTIES OF MATTER |
| Chapter12: KINETIC THEORY |
| Chapter13: OSCILLATIONS |
| Chapter14: WAVES |
| CBSE Class 11 Mathematics chapter |
| Chapter1: SETS |
| Chapter2: RELATIONS AND FUNCTIONS |
| Chapter3: TRIGONOMETRIC FUNCTIONS |
| Chapter4: COMPLEX NUMBER AND QUADRATIC EQUATIONS |
| Chapter5: LINEAR INEQUALITIES |
| Chapter6: PERMUTATIONS AND COMBINATIONS |
| Chapter7: BINOMIAL THEOREM |
| Chapter8: SEQUENCES AND SERIES |
| Chapter9: STRAIGHT LINES |
| Chapter10: CONIC SECTIONS |
| Chapter11: INTRODUCTION TO THREE-DIMENSIONAL GEOMETRY |
| Chapter12: LIMITS AND DERIVATIVES |
| Chapter13: STATISTICS |
| Chapter14: PROBABILITY |
| Class 8 Link soon |
| Class 9 Link soon |
| Class 10 Link soon |
| Class 12 Link soon |
Leave a Reply